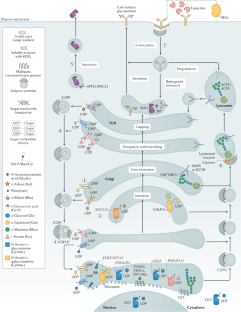
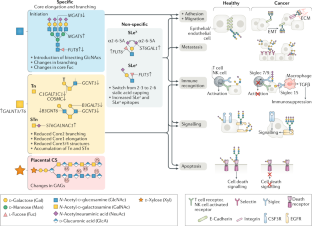
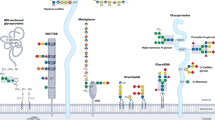
Global view of human protein glycosylation pathways and functions
Glycosylation is the most abundant and diverse form of post-translational modification of proteins that is common to all eukaryotic cells. Enzymatic glycosylation of proteins involves a complex metabolic network and different types of glycosylation pathways that orchestrate enormous amplification of the proteome in producing diversity of proteoforms and its biological functions. The tremendous structural diversity of glycans attached to proteins poses analytical challenges that limit exploration of specific functions of glycosylation. Major advances in quantitative transcriptomics, proteomics and nuclease-based gene editing are now opening new global ways to explore protein glycosylation through analysing and targeting enzymes involved in glycosylation processes. In silico models predicting cellular glycosylation capacities and glycosylation outcomes are emerging, and refined maps of the glycosylation pathways facilitate genetic approaches to address functions of the vast glycoproteome. These approaches apply commonly available cell biology tools, and we predict that use of (single-cell) transcriptomics, genetic screens, genetic engineering of cellular glycosylation capacities and custom design of glycoprotein therapeutics are advancements that will ignite wider integration of glycosylation in general cell biology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
206,07 € per year
only 17,17 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Genetics of glycosylation in mammalian development and disease
Article 09 May 2024
Glycosylation: mechanisms, biological functions and clinical implications
Article Open access 05 August 2024
Glycoproteomics
Article 23 June 2022
References
- Fournet, M., Bonté, F. & Desmoulière, A. Glycation damage: a possible hub for major pathophysiological disorders and aging. Aging Dis.9, 880–900 (2018). PubMedPubMed CentralGoogle Scholar
- Steentoft, C. et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J.32, 1478–1488 (2013). This paper presents a deep analysis of the human GalNAc-type O-glycoproteome. CASPubMedPubMed CentralGoogle Scholar
- Zielinska, D. F., Gnad, F., Wiśniewski, J. R. & Mann, M. Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell141, 897–907 (2010). This paper presents a deep analysis of N-glycosites in the human proteome. CASPubMedGoogle Scholar
- Hart, G. W. Nutrient regulation of signaling and transcription. J. Biol. Chem.294, 2211–2231 (2019). CASPubMedPubMed CentralGoogle Scholar
- Hart, G. W. et al. Glycosylation of Nuclear and Cytoplasmic Proteins is as Abundant and as Dynamic as Phosphorylation. in Glyco- and Cellbiology (eds Wieland, F. & Reutter, W.) 91–103 (Springer, 1994).
- Aebersold, R. et al. How many human proteoforms are there? Nat. Chem. Biol.14, 206–214 (2018). CASPubMedPubMed CentralGoogle Scholar
- Varki, A. Biological roles of glycans. Glycobiology27, 3–49 (2017). CASPubMedGoogle Scholar
- Spiro, R. G. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology12, 43R–56R (2002). CASPubMedGoogle Scholar
- Cummings, R. D. The repertoire of glycan determinants in the human glycome. Mol. Biosyst.5, 1087–1104 (2009). CASPubMedGoogle Scholar
- Joshi, H. J. et al. SnapShot: O-glycosylation pathways across kingdoms. Cell172, 632–632.e2 (2018). CASPubMedGoogle Scholar
- Springer, S. A. & Gagneux, P. Glycomics: revealing the dynamic ecology and evolution of sugar molecules. J. Proteom.135, 90–100 (2016). CASGoogle Scholar
- Chou, H. H. et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc. Natl Acad. Sci. USA95, 11751–11756 (1998). CASPubMedPubMed CentralGoogle Scholar
- Larsen, R. D., Rivera-Marrero, C. A., Ernst, L. K., Cummings, R. D. & Lowe, J. B. Frameshift and nonsense mutations in a human genomic sequence homologous to a murine UDP-Gal:β- d -Gal(1,4)- d -GlcNAc α(1,3)-galactosyltransferase cDNA. J. Biol. Chem.265, 7055–7061 (1990). CASPubMedGoogle Scholar
- Christiansen, D. et al. Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol.6, e172 (2008). PubMedPubMed CentralGoogle Scholar
- Stanley, P. What have we learned from glycosyltransferase knockouts in mice? J. Mol. Biol.428, 3166–3182 (2016). CASPubMedPubMed CentralGoogle Scholar
- Lowe, J. B. & Marth, J. D. A genetic approach to mammalian glycan function. Annu. Rev. Biochem.72, 643–691 (2003). CASPubMedGoogle Scholar
- Freeze, H. H., Chong, J. X., Bamshad, M. J. & Ng, B. G. Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am. J. Hum. Genet.94, 161–175 (2014). CASPubMedPubMed CentralGoogle Scholar
- Jaeken, J. & Péanne, R. What is new in CDG? J. Inherit. Metab. Dis.40, 569–586 (2017). CASPubMedGoogle Scholar
- Moremen, K. W., Tiemeyer, M. & Nairn, A. V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol.13, 448–462 (2012). CASPubMedPubMed CentralGoogle Scholar
- Joshi, H. J. et al. Glycosyltransferase genes that cause monogenic congenital disorders of glycosylation are distinct from glycosyltransferase genes associated with complex diseases. Glycobiology28, 284–294 (2018). CASPubMedPubMed CentralGoogle Scholar
- Hansen, L. et al. A mutation map for human glycoside hydrolase genes. Glycobiology30, 500–515 (2020). PubMedPubMed CentralGoogle Scholar
- Cummings, R. D. & Pierce, J. M. The challenge and promise of glycomics. Chem. Biol.21, 1–15 (2014). CASPubMedPubMed CentralGoogle Scholar
- Kornfeld, R. & Kornfeld, S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem.54, 631–664 (1985). CASPubMedGoogle Scholar
- Narimatsu, H. Human glycogene cloning: focus on β3-glycosyltransferase and β4-glycosyltransferase families. Curr. Opin. Struct. Biol.16, 567–575 (2006). CASPubMedGoogle Scholar
- Bennett, E. P. et al. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology22, 736–756 (2012). CASPubMedGoogle Scholar
- Tsuji, S., Datta, A. K. & Paulson, J. C. Systematic nomenclature for sialyltransferases. Glycobiology6, v–vii (1996). CASPubMedGoogle Scholar
- Larsen, I. S. B. et al. Discovery of an O-mannosylation pathway selectively serving cadherins and protocadherins. Proc. Natl Acad. Sci. USA114, 11163–11168 (2017). PubMedPubMed CentralGoogle Scholar
- Yoshida-Moriguchi, T. & Campbell, K. P. Matriglycan: a novel polysaccharide that links dystroglycan to the basement membrane. Glycobiology25, 702–713 (2015). CASPubMedPubMed CentralGoogle Scholar
- Praissman, J. L. et al. The functional O-mannose glycan on α-dystroglycan contains a phospho-ribitol primed for matriglycan addition. eLife5, e14473 (2016). PubMedPubMed CentralGoogle Scholar
- Hirata, T. et al. Identification of a Golgi GPI-N-acetylgalactosamine transferase with tandem transmembrane regions in the catalytic domain. Nat. Commun.9, 405 (2018). PubMedPubMed CentralGoogle Scholar
- Cejas, R. B., Lorenz, V., Garay, Y. C. & Irazoqui, F. J. Biosynthesis of O-N-acetylgalactosamine glycans in the human cell nucleus. J. Biol. Chem.294, 2997–3011 (2019). CASPubMedGoogle Scholar
- Tu, L., Chen, L. & Banfield, D. K. A conserved N-terminal arginine-motif in GOLPH3-family proteins mediates binding to coatomer. Traffic13, 1496–1507 (2012). CASPubMedGoogle Scholar
- Liu, L., Doray, B. & Kornfeld, S. Recycling of Golgi glycosyltransferases requires direct binding to coatomer. Proc. Natl Acad. Sci. USA115, 8984–8989 (2018). CASPubMedPubMed CentralGoogle Scholar
- Kuhn, P.-H. et al. Secretome analysis identifies novel signal peptide peptidase-like 3 (Sppl3) substrates and reveals a role of Sppl3 in multiple Golgi glycosylation pathways. Mol. Cell. Proteom.14, 1584–1598 (2015). CASGoogle Scholar
- Schjoldager, K. T.-B. G. et al. A systematic study of site-specific GalNAc-type O-glycosylation modulating proprotein convertase processing. J. Biol. Chem.286, 40122–40132 (2011). CASPubMedGoogle Scholar
- Shifley, E. T. & Cole, S. E. Lunatic fringe protein processing by proprotein convertases may contribute to the short protein half-life in the segmentation clock. Biochim. Biophys. Acta1783, 2384–2390 (2008). CASPubMedGoogle Scholar
- Paulson, J. C. & Colley, K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J. Biol. Chem.264, 17615–17618 (1989). CASPubMedGoogle Scholar
- Liefhebber, J. M., Punt, S., Spaan, W. J. & van Leeuwen, H. C. The human collagen β(1-O)galactosyltransferase, GLT25D1, is a soluble endoplasmic reticulum localized protein. BMC Cell Biol.11, 33 (2010). PubMedPubMed CentralGoogle Scholar
- Harvey, B. M. & Haltiwanger, R. S. Regulation of Notch function by O-glycosylation. Adv. Exp. Med. Biol.1066, 59–78 (2018). CASPubMedGoogle Scholar
- Ogawa, M. et al. GTDC2 modifies O-mannosylated α-dystroglycan in the endoplasmic reticulum to generate N-acetyl glucosamine epitopes reactive with CTD110.6 antibody. Biochem. Biophys. Res. Commun.440, 88–93 (2013). CASPubMedGoogle Scholar
- Snider, M. D. & Rogers, O. C. Intracellular movement of cell surface receptors after endocytosis: resialylation of asialo-transferrin receptor in human erythroleukemia cells. J. Cell Biol.100, 826–834 (1985). CASPubMedGoogle Scholar
- Duncan, J. R. & Kornfeld, S. Intracellular movement of two mannose 6-phosphate receptors: return to the Golgi apparatus. J. Cell Biol.106, 617–628 (1988). CASPubMedGoogle Scholar
- Litvinov, S. V. & Hilkens, J. The epithelial sialomucin, episialin, is sialylated during recycling. J. Biol. Chem.268, 21364–21371 (1993). CASPubMedGoogle Scholar
- Razawi, H. et al. Evidence for Core 2 to Core 1 O-glycan remodeling during the recycling of MUC1. Glycobiology23, 935–45 (2013). CASPubMedPubMed CentralGoogle Scholar
- Gilmour, A. M. et al. A novel epidermal growth factor receptor-signaling platform and its targeted translation in pancreatic cancer. Cell Signal.25, 2587–2603 (2013). CASPubMedGoogle Scholar
- Haxho, F., Neufeld, R. J. & Szewczuk, M. R. Neuraminidase-1: a novel therapeutic target in multistage tumorigenesis. Oncotarget7, 40860–40881 (2016). PubMedPubMed CentralGoogle Scholar
- Lillehoj, E. P. et al. NEU1 sialidase expressed in human airway epithelia regulates epidermal growth factor receptor (EGFR) and MUC1 protein signaling. J. Biol. Chem.287, 8214–8231 (2012). CASPubMedPubMed CentralGoogle Scholar
- Welch, L. G. & Munro, S. A tale of short tails, through thick and thin: investigating the sorting mechanisms of Golgi enzymes. FEBS Lett.593, 2452–2465 (2019). CASPubMedGoogle Scholar
- Kellokumpu, S., Hassinen, A. & Glumoff, T. Glycosyltransferase complexes in eukaryotes: long-known, prevalent but still unrecognized. Cell. Mol. Life Sci.73, 305–325 (2016). CASPubMedGoogle Scholar
- Moremen, K. W. & Haltiwanger, R. S. Emerging structural insights into glycosyltransferase-mediated synthesis of glycans. Nat. Chem. Biol.15, 853–864 (2019). CASPubMedPubMed CentralGoogle Scholar
- de Las Rivas, M., Lira-Navarrete, E., Gerken, T. A. & Hurtado-Guerrero, R. Polypeptide GalNAc-Ts: from redundancy to specificity. Curr. Opin. Struct. Biol.56, 87–96 (2019). PubMedGoogle Scholar
- Taujale, R. et al. Deep evolutionary analysis reveals the design principles of fold A glycosyltransferases. eLife9, e54532 (2020). CASPubMedPubMed CentralGoogle Scholar
- Kinoshita, T. & Fujita, M. Biosynthesis of GPI-anchored proteins: special emphasis on GPI lipid remodeling. J. Lipid Res.57, 6–24 (2016). CASPubMedPubMed CentralGoogle Scholar
- Wild, R. et al. Structure of the yeast oligosaccharyltransferase complex gives insight into eukaryotic N-glycosylation. Science359, 545–550 (2018). CASPubMedGoogle Scholar
- Ruiz-Canada, C., Kelleher, D. J. & Gilmore, R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell136, 272–283 (2009). CASPubMedPubMed CentralGoogle Scholar
- Cherepanova, N. A. & Gilmore, R. Mammalian cells lacking either the cotranslational or posttranslocational oligosaccharyltransferase complex display substrate-dependent defects in asparagine linked glycosylation. Sci. Rep.6, 20946 (2016). CASPubMedPubMed CentralGoogle Scholar
- Ramírez, A. S., Kowal, J. & Locher, K. P. Cryo–electron microscopy structures of human oligosaccharyltransferase complexes OST-A and OST-B. Science366, 1372–1375 (2019). PubMedGoogle Scholar
- Harada, Y., Masahara-Negishi, Y. & Suzuki, T. Cytosolic-free oligosaccharides are predominantly generated by the degradation of dolichol-linked oligosaccharides in mammalian cells. Glycobiology25, 1196–1205 (2015). CASPubMedGoogle Scholar
- Lu, H. et al. Mammalian STT3A/B oligosaccharyltransferases segregate N-glycosylation at the translocon from lipid-linked oligosaccharide hydrolysis. Proc. Natl Acad. Sci. USA115, 9557–9562 (2018). CASPubMedPubMed CentralGoogle Scholar
- Shan, A. et al. Polypeptide N-acetylgalactosaminyltransferase 18 non-catalytically regulates the ER homeostasis and O-glycosylation. Biochim. Biophys. Acta Gen. Subj.1863, 870–882 (2019). CASPubMedGoogle Scholar
- Joshi, H. J. et al. GlycoDomainViewer: a bioinformatics tool for contextual exploration of glycoproteomes. Glycobiology28, 131–136 (2018). CASPubMedGoogle Scholar
- Schjoldager, K. T. et al. Deconstruction of O-glycosylation–GalNAc-T isoforms direct distinct subsets of the O-glycoproteome. EMBO Rep.16, 1713–1722 (2015). CASPubMedPubMed CentralGoogle Scholar
- Narimatsu, Y. et al. Exploring regulation of protein O-glycosylation in isogenic human HEK293 cells by differential O-glycoproteomics. Mol. Cell. Proteom.18, 1396–1409 (2019). CASGoogle Scholar
- Bagdonaite, I. et al. O-glycan initiation directs distinct biological pathways and controls epithelial differentiation. EMBO Rep.21, e48885 (2020). CASPubMedPubMed CentralGoogle Scholar
- Wang, S. et al. Site-specific O-glycosylation of members of the low-density lipoprotein receptor superfamily enhances ligand interactions. J. Biol. Chem.293, 7408–7422 (2018). This paper describes a role of site-specific O-glycosylation in regulation of the affinity of LDLR-related receptors. CASPubMedPubMed CentralGoogle Scholar
- Tagliabracci, V. S. et al. A single kinase generates the majority of the secreted phosphoproteome. Cell161, 1619–1632 (2015). CASPubMedPubMed CentralGoogle Scholar
- Tagliabracci, V. S. et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc. Natl Acad. Sci. USA111, 5520–5 (2014). This paper is the first demonstration of cross-talk between extracellular protein phosphorylation and O-glycosylation. CASPubMedPubMed CentralGoogle Scholar
- Bordoli, M. R. et al. A secreted tyrosine kinase acts in the extracellular environment. Cell158, 1033–1044 (2014). CASPubMedPubMed CentralGoogle Scholar
- Mehta, A. Y., Heimburg-Molinaro, J., Cummings, R. D. & Goth, C. K. Emerging patterns of tyrosine sulfation and O-glycosylation cross-talk and co-localization. Curr. Opin. Struct. Biol.62, 102–111 (2020). CASPubMedPubMed CentralGoogle Scholar
- Yu, H. & Takeuchi, H. Protein O-glucosylation: another essential role of glucose in biology. Curr. Opin. Struct. Biol.56, 64–71 (2019). CASPubMedGoogle Scholar
- Holdener, B. C. & Haltiwanger, R. S. Protein O-fucosylation: structure and function. Curr. Opin. Struct. Biol.56, 78–86 (2019). CASPubMedPubMed CentralGoogle Scholar
- Ogawa, M. & Okajima, T. Structure and function of extracellular O-GlcNAc. Curr. Opin. Struct. Biol.56, 72–77 (2019). CASPubMedGoogle Scholar
- Takeuchi, H. et al. Two novel protein O-glucosyltransferases that modify sites distinct from POGLUT1 and affect Notch trafficking and signaling. Proc. Natl Acad. Sci. USA115, E8395–E8402 (2018). This paper describes the identification and differential functions of POGLUT isoenzymes in glycosylation of NOTCH EGF-like repeats and their regulation of NOTCH functions. CASPubMedPubMed CentralGoogle Scholar
- Sakaidani, Y. et al. O-linked-N-acetylglucosamine modification of mammalian Notch receptors by an atypical O-GlcNAc transferase Eogt1. Biochem. Biophys. Res. Commun.419, 14–19 (2012). CASPubMedGoogle Scholar
- Manya, H. et al. Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity. Proc. Natl Acad. Sci. USA101, 500–505 (2004). CASPubMedGoogle Scholar
- Neubert, P. et al. Mapping the O-mannose glycoproteome in Saccharomyces cerevisiae. Mol. Cell. Proteom.15, 1323–1337 (2016). CASGoogle Scholar
- Shcherbakova, A., Tiemann, B., Buettner, F. F. R. & Bakker, H. Distinct C-mannosylation of netrin receptor thrombospondin type 1 repeats by mammalian DPY19L1 and DPY19L3. Proc. Natl Acad. Sci. USA114, 2574–2579 (2017). This paper describes the DPY19L isoenzymes directing C-mannosylation and identifies distinct differences in their substrate preferences. CASPubMedPubMed CentralGoogle Scholar
- Roch, C., Kuhn, J., Kleesiek, K. & Götting, C. Differences in gene expression of human xylosyltransferases and determination of acceptor specificities for various proteoglycans. Biochem. Biophys. Res. Commun.391, 685–691 (2010). CASPubMedGoogle Scholar
- Noborn, F. et al. Identification of chondroitin sulfate linkage region glycopeptides reveals prohormones as a novel class of proteoglycans. Mol. Cell. Proteom.14, 41–49 (2015). CASGoogle Scholar
- Hennet, T. Collagen glycosylation. Curr. Opin. Struct. Biol.56, 131–138 (2019). CASPubMedGoogle Scholar
- Scietti, L. et al. Molecular architecture of the multifunctional collagen lysyl hydroxylase and glycosyltransferase LH3. Nat. Commun.9, 3163 (2018). PubMedPubMed CentralGoogle Scholar
- Slawson, C. & Hart, G. W. O-GlcNAc signalling: implications for cancer cell biology. Nat. Rev. Cancer11, 678–684 (2011). CASPubMedPubMed CentralGoogle Scholar
- Lazarus, M. B., Nam, Y., Jiang, J., Sliz, P. & Walker, S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature469, 564–567 (2011). CASPubMedPubMed CentralGoogle Scholar
- Tsuji, S., Datta, A. K. & Paulson, J. C. Systematic nomenclature for sialyltransferases. Glycobiology6, v–vii (1996). CASPubMedGoogle Scholar
- Oriol, R., Mollicone, R., Cailleau, A., Balanzino, L. & Breton, C. Divergent evolution of fucosyltransferase genes from vertebrates, invertebrates, and bacteria. Glycobiology9, 323–334 (1999). CASPubMedGoogle Scholar
- Nagae, M., Yamaguchi, Y., Taniguchi, N. & Kizuka, Y. 3D structure and function of glycosyltransferases involved in N-glycan maturation. Int J. Mol. Sci.21, 437 (2020). CASPubMed CentralGoogle Scholar
- Honke, K. & Taniguchi, N. Sulfotransferases and sulfated oligosaccharides. Med. Res. Rev.22, 637–654 (2002). CASPubMedGoogle Scholar
- Esko, J. D. & Selleck, S. B. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem.71, 435–471 (2002). CASPubMedGoogle Scholar
- Mikami, T. & Kitagawa, H. Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta1830, 4719–4733 (2013). CASPubMedGoogle Scholar
- Kitayama, K., Hayashida, Y., Nishida, K. & Akama, T. O. Enzymes responsible for synthesis of corneal keratan sulfate glycosaminoglycans. J. Biol. Chem.282, 30085–30096 (2007). CASPubMedGoogle Scholar
- Yoshida-Moriguchi, T. et al. SGK196 is a glycosylation-specific O-mannose kinase required for dystroglycan function. Science341, 896–899 (2013). CASPubMedGoogle Scholar
- Sheikh, M. O., Halmo, S. M. & Wells, L. Recent advancements in understanding mammalian O-mannosylation. Glycobiology27, 806–819 (2017). CASPubMedPubMed CentralGoogle Scholar
- Koike, T., Izumikawa, T., Tamura, J.-I. & Kitagawa, H. FAM20B is a kinase that phosphorylates xylose in the glycosaminoglycan–protein linkage region. Biochem. J.421, 157–162 (2009). CASPubMedGoogle Scholar
- Wen, J. et al. Xylose phosphorylation functions as a molecular switch to regulate proteoglycan biosynthesis. Proc. Natl Acad. Sci. USA111, 15723–15728 (2014). CASPubMedPubMed CentralGoogle Scholar
- Duan, S. & Paulson, J. C. Siglecs as immune cell checkpoints in disease. Annu. Rev. Immunol.38, 365–395 (2020). CASPubMedGoogle Scholar
- Baumann, A.-M. T. et al. 9-O-Acetylation of sialic acids is catalysed by CASD1 via a covalent acetyl-enzyme intermediate. Nat. Commun.6, 7673 (2015). CASPubMedGoogle Scholar
- Orizio, F. et al. Human sialic acid acetyl esterase: towards a better understanding of a puzzling enzyme. Glycobiology25, 992–1006 (2015). CASPubMedGoogle Scholar
- Vlasak, R., Luytjes, W., Spaan, W. & Palese, P. Human and bovine coronaviruses recognize sialic acid-containing receptors similar to those of influenza C viruses. Proc. Natl Acad. Sci. USA85, 4526–4529 (1988). CASPubMedPubMed CentralGoogle Scholar
- Barb, A. W. & Prestegard, J. H. NMR analysis demonstrates immunoglobulin G N-glycans are accessible and dynamic. Nat. Chem. Biol.7, 147–153 (2011). CASPubMedPubMed CentralGoogle Scholar
- Krapp, S., Mimura, Y., Jefferis, R., Huber, R. & Sondermann, P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J. Mol. Biol.325, 979–989 (2003). CASPubMedGoogle Scholar
- Bowden, T. A. et al. Chemical and structural analysis of an antibody folding intermediate trapped during glycan biosynthesis. J. Am. Chem. Soc.134, 17554–17563 (2012). CASPubMedPubMed CentralGoogle Scholar
- Patel, K. R., Roberts, J. T. & Barb, A. W. Multiple variables at the leukocyte cell surface impact Fcγ receptor-dependent mechanisms. Front. Immunol.10, 223 (2019). CASPubMedPubMed CentralGoogle Scholar
- Ye, Z., Mao, Y., Clausen, H. & Vakhrushev, S. Y. Glyco-DIA: a method for quantitative O-glycoproteomics with in silico-boosted glycopeptide libraries. Nat. Methods16, 902–910 (2019). CASPubMedGoogle Scholar
- Kornfeld, S. Trafficking of lysosomal enzymes in normal and disease states. J. Clin. Invest.77, 1–6 (1986). CASPubMedPubMed CentralGoogle Scholar
- Reitman, M. L. & Kornfeld, S. Lysosomal enzyme targeting. N-Acetylglucosaminylphosphotransferase selectively phosphorylates native lysosomal enzymes. J. Biol. Chem.256, 11977–11980 (1981). CASPubMedGoogle Scholar
- Schnaar, R. L., Gerardy-Schahn, R. & Hildebrandt, H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev.94, 461–518 (2014). PubMedPubMed CentralGoogle Scholar
- Bhide, G. P., Prehna, G., Ramirez, B. E. & Colley, K. J. The polybasic region of the polysialyltransferase ST8Sia-IV binds directly to the neural cell adhesion molecule, NCAM. Biochemistry56, 1504–1517 (2017). CASPubMedGoogle Scholar
- Bhide, G. P., Fernandes, N. R. J. & Colley, K. J. Sequence requirements for neuropilin-2 recognition by ST8SiaIV and polysialylation of its O-glycans. J. Biol. Chem.291, 9444–9457 (2016). CASPubMedPubMed CentralGoogle Scholar
- Marcus, D. M. & Cass, L. E. Glycosphingolipids with Lewis blood group activity: uptake by human erythrocytes. Science164, 553–555 (1969). CASPubMedGoogle Scholar
- Parry, S. et al. The sperm agglutination antigen-1 (SAGA-1) glycoforms of CD52 are O-glycosylated. Glycobiology17, 1120–1126 (2007). CASPubMedGoogle Scholar
- Wandall, H. H. et al. The origin and function of platelet glycosyltransferases. Blood120, 626–635 (2012). CASPubMedPubMed CentralGoogle Scholar
- Manhardt, C. T., Punch, P. R., Dougher, C. W. L. & Lau, J. T. Y. Extrinsic sialylation is dynamically regulated by systemic triggers in vivo. J. Biol. Chem.292, 13514–13520 (2017). CASPubMedPubMed CentralGoogle Scholar
- Jones, M. B. et al. B-cell-independent sialylation of IgG. Proc. Natl Acad. Sci. USA113, 7207–7212 (2016). CASPubMedPubMed CentralGoogle Scholar
- Irons, E. E. et al. B cells suppress medullary granulopoiesis by an extracellular glycosylation-dependent mechanism. eLife8, e47328 (2019). CASPubMedPubMed CentralGoogle Scholar
- Zhang, Q. et al. Transfer of functional cargo in exomeres. Cell Rep.27, 940–954.e6 (2019). CASPubMedPubMed CentralGoogle Scholar
- Lu, Q., Li, S. & Shao, F. Sweet talk: protein glycosylation in bacterial interaction with the host. Trends Microbiol.23, 630–641 (2015). CASPubMedGoogle Scholar
- El Qaidi, S. et al. NleB/SseK effectors from Citrobacter rodentium, Escherichia coli, and Salmonella enterica display distinct differences in host substrate specificity. J. Biol. Chem.292, 11423–11430 (2017). CASPubMedPubMed CentralGoogle Scholar
- Li, S. et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature501, 242–246 (2013). CASPubMedGoogle Scholar
- Helenius, A. & Aebi, M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem.73, 1019–1049 (2004). CASPubMedGoogle Scholar
- Vasudevan, D., Takeuchi, H., Johar, S. S., Majerus, E. & Haltiwanger, R. S. Peters plus syndrome mutations disrupt a noncanonical ER quality-control mechanism. Curr. Biol.25, 286–295 (2015). CASPubMedGoogle Scholar
- Takeuchi, H. et al. O-Glycosylation modulates the stability of epidermal growth factor-like repeats and thereby regulates Notch trafficking. J. Biol. Chem.292, 15964–15973 (2017). CASPubMedPubMed CentralGoogle Scholar
- Ogawa, M., Tashima, Y., Sakaguchi, Y., Takeuchi, H. & Okajima, T. Contribution of extracellular O-GlcNAc to the stability of folded epidermal growth factor-like domains and Notch1 trafficking. Biochem. Biophys. Res. Commun.526, 184–190 (2020). CASPubMedGoogle Scholar
- Goth, C. K., Vakhrushev, S. Y., Joshi, H. J., Clausen, H. & Schjoldager, K. T. Fine-tuning limited proteolysis: a major role for regulated site-specific O-glycosylation. Trends Biochem. Sci.43, 269–284 (2018). CASPubMedGoogle Scholar
- Goth, C. K. et al. A systematic study of modulation of ADAM-mediated ectodomain shedding by site-specific O-glycosylation. Proc. Natl Acad. Sci. USA112, 14623–14628 (2015). CASPubMedPubMed CentralGoogle Scholar
- Goth, C. K. et al. Site-specific O-glycosylation by polypeptide N-acetylgalactosaminyltransferase 2 (GalNAc-transferase T2) co-regulates β1-adrenergic receptor N-terminal cleavage. J. Biol. Chem.292, 4714–4726 (2017). CASPubMedPubMed CentralGoogle Scholar
- Hansen, L. H. et al. Discovery of O-glycans on atrial natriuretic peptide (ANP) that affect both its proteolytic degradation and potency at its cognate receptor. J. Biol. Chem.294, 12567–12578 (2019). CASPubMedPubMed CentralGoogle Scholar
- Madsen, T. D. et al. An atlas of O-linked glycosylation on peptide hormones reveals diverse biological roles. Nat. Commun.11, 4033 (2020). CASPubMedPubMed CentralGoogle Scholar
- Hintze, J. et al. Probing the contribution of individual polypeptide GalNAc-transferase isoforms to the O-glycoproteome by inducible expression in isogenic cell lines. J. Biol. Chem.293, 19064–19077 (2018). CASPubMedPubMed CentralGoogle Scholar
- Kato, K. et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem.281, 18370–18377 (2006). CASPubMedGoogle Scholar
- Takashi, Y. et al. Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proc. Natl Acad. Sci. USA116, 11418–11427 (2019). CASPubMedPubMed CentralGoogle Scholar
- Larsen, I. S. B., Narimatsu, Y., Clausen, H., Joshi, H. J. & Halim, A. Multiple distinct O-mannosylation pathways in eukaryotes. Curr. Opin. Struct. Biol.56, 171–178 (2019). CASPubMedGoogle Scholar
- Rexach, J. E. et al. Dynamic O-GlcNAc modification regulates CREB-mediated gene expression and memory formation. Nat. Chem. Biol.8, 253–261 (2012). CASPubMedPubMed CentralGoogle Scholar
- Tarbet, H. J. et al. Site-specific glycosylation regulates the form and function of the intermediate filament cytoskeleton. eLife7, e31807 (2018). PubMedPubMed CentralGoogle Scholar
- Dennis, J. W. & Brewer, C. F. Density-dependent lectin-glycan interactions as a paradigm for conditional regulation by posttranslational modifications. Mol. Cell. Proteom.12, 913–20 (2013). CASGoogle Scholar
- Granovsky, M. et al. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med.6, 306–312 (2000). CASPubMedGoogle Scholar
- Martínez Allo, V. C. et al. Suppression of age-related salivary gland autoimmunity by glycosylation-dependent galectin-1-driven immune inhibitory circuits. Proc. Natl Acad. Sci. USA117, 6630–6639 (2020). PubMedPubMed CentralGoogle Scholar
- Demetriou, M., Nabi, I. R., Coppolino, M., Dedhar, S. & Dennis, J. W. Reduced contact-inhibition and substratum adhesion in epithelial cells expressing GlcNAc-transferase V. J. Cell Biol.130, 383–392 (1995). CASPubMedGoogle Scholar
- Nakano, M. et al. Bisecting GlcNAc is a general suppressor of terminal modification of N-glycan. Mol. Cell. Proteom.18, 2044–2057 (2019). Google Scholar
- Isaji, T. et al. Introduction of bisecting GlcNAc into integrin α5β1 reduces ligand binding and down-regulates cell adhesion and cell migration. J. Biol. Chem.279, 19747–19754 (2004). CASPubMedGoogle Scholar
- Wang, X. et al. Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J. Biol. Chem.281, 2572–2577 (2006). CASPubMedGoogle Scholar
- Liang, W. et al. Core fucosylation of the T cell receptor is required for T cell activation. Front. Immunol.9, 78 (2018). PubMedPubMed CentralGoogle Scholar
- Shields, R. L. et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J. Biol. Chem.277, 26733–26740 (2002). This paper identifies core fucose on the N-glycans of IgG1 in regulation of antibody effector functions. CASPubMedGoogle Scholar
- Nguyen, J. T. et al. CD45 modulates galectin-1-induced T cell death: regulation by expression of Core 2 O-glycans. J. Immunol.167, 5697–5707 (2001). CASPubMedGoogle Scholar
- Lee, S. H. et al. Core2 O-glycan structure is essential for the cell surface expression of sucrase isomaltase and dipeptidyl peptidase-IV during intestinal cell differentiation. J. Biol. Chem.285, 37683–37692 (2010). CASPubMedPubMed CentralGoogle Scholar
- Halmo, S. M. et al. Protein O-linked mannose β-1,4-N-acetylglucosaminyl-transferase 2 (POMGNT2) is a gatekeeper enzyme for functional glycosylation of α-dystroglycan. J. Biol. Chem.292, 2101–2109 (2017). This paper describes the peptide substrate selectivity of the ER-located POMGNT2 that determines theO-Man glycosites that proceed towards biosynthesis of the elaborated matriglycan.CASPubMedGoogle Scholar
- Varki, A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature446, 1023–1029 (2007). CASPubMedGoogle Scholar
- Cohen, M. & Varki, A. Modulation of glycan recognition by clustered saccharide patches. Int. Rev. Cell Mol. Biol.308, 75–125 (2014). CASPubMedGoogle Scholar
- Giannini, S. et al. β4GALT1 controls β1 integrin function to govern thrombopoiesis and hematopoietic stem cell homeostasis. Nat. Commun.11, 356 (2020). CASPubMedPubMed CentralGoogle Scholar
- Hennet, T., Chui, D., Paulson, J. C. & Marth, J. D. Immune regulation by the ST6Gal sialyltransferase. Proc. Natl Acad. Sci. USA95, 4504–4509 (1998). CASPubMedPubMed CentralGoogle Scholar
- Comelli, E. M. et al. Activation of murine CD4 + and CD8 + T lymphocytes leads to dramatic remodeling of N-linked glycans. J. Immunol.177, 2431–2440 (2006). CASPubMedGoogle Scholar
- Priatel, J. J. et al. The ST3Gal-I sialyltransferase controls CD8 + T lymphocyte homeostasis by modulating O-glycan biosynthesis. Immunity12, 273–283 (2000). CASPubMedGoogle Scholar
- Ohtsubo, K. & Marth, J. D. Glycosylation in cellular mechanisms of health and disease. Cell126, 855–867 (2006). CASPubMedGoogle Scholar
- Mereiter, S., Balmaña, M., Campos, D., Gomes, J. & Reis, C. A. Glycosylation in the era of cancer-targeted therapy: where are we heading? Cancer Cell36, 6–16 (2019). CASPubMedGoogle Scholar
- Ng, B. G. & Freeze, H. H. Perspectives on glycosylation and its congenital disorders. Trends Genet.34, 466–476 (2018). CASPubMedPubMed CentralGoogle Scholar
- Zilmer, M. et al. Novel congenital disorder of O-linked glycosylation caused by loss of function of GALNT2. Brain143, 1114–1126 (2020). PubMedPubMed CentralGoogle Scholar
- Topaz, O. et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat. Genet.36, 579–581 (2004). CASPubMedGoogle Scholar
- Tian, E. et al. Galnt11 regulates kidney function by glycosylating the endocytosis receptor megalin to modulate ligand binding. Proc. Natl Acad. Sci. USA116, 25196–25202 (2019). CASPubMedPubMed CentralGoogle Scholar
- Hansen, L. et al. A glycogene mutation map for discovery of diseases of glycosylation. Glycobiology25, 211–224 (2015). CASPubMedGoogle Scholar
- Willer, C. J. et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet.40, 161–169 (2008). CASPubMedPubMed CentralGoogle Scholar
- Khetarpal, S. A. et al. Loss of function of GALNT2 lowers high-density lipoproteins in humans, nonhuman primates, and rodents. Cell Metab.24, 234–245 (2016). This paper describes validation of the first glycosyltransferase gene implicated as a GWAS candidate. CASPubMedPubMed CentralGoogle Scholar
- Roman, T. S. et al. Multiple hepatic regulatory variants at the GALNT2 GWAS locus associated with high-density lipoprotein cholesterol. Am. J. Hum. Genet.97, 801–815 (2015). CASPubMedPubMed CentralGoogle Scholar
- Cavalli, M., Pan, G., Nord, H. & Wadelius, C. Looking beyond GWAS: allele-specific transcription factor binding drives the association of GALNT2 to HDL-C plasma levels. Lipids Health Dis.15, 18 (2016). PubMedPubMed CentralGoogle Scholar
- Duncan, E. L. et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet.7, e1001372 (2011). CASPubMedPubMed CentralGoogle Scholar
- de Las Rivas, M. et al. Molecular basis for fibroblast growth factor 23 O-glycosylation by GalNAc-T3. Nat. Chem. Biol.16, 351–360 (2020). PubMedGoogle Scholar
- Taniguchi, N. & Kizuka, Y. Glycans and cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics. Adv. Cancer Res.126, 11–51 (2015). CASPubMedGoogle Scholar
- Schultz, M. J., Swindall, A. F. & Bellis, S. L. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev.31, 501–518 (2012). CASPubMedPubMed CentralGoogle Scholar
- Christiansen, M. N. et al. Cell surface protein glycosylation in cancer. Proteomics14, 525–546 (2014). CASPubMedGoogle Scholar
- Ju, T. et al. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res.68, 1636–46 (2008). CASPubMedGoogle Scholar
- Sun, X., Ju, T. & Cummings, R. D. Differential expression of Cosmc, T-synthase and mucins in Tn-positive colorectal cancers. BMC Cancer18, 827 (2018). PubMedPubMed CentralGoogle Scholar
- Radhakrishnan, P. et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc. Natl Acad. Sci. USA111, E4066–E4075 (2014). CASPubMedPubMed CentralGoogle Scholar
- Fernandez, A. J. et al. The structure of the colorectal cancer-associated enzyme GalNAc-T12 reveals how nonconserved residues dictate its function. Proc. Natl Acad. Sci. USA116, 20404–20410 (2019). CASPubMedPubMed CentralGoogle Scholar
- Büll, C., Stoel, M. A., den Brok, M. H. & Adema, G. J. Sialic acids sweeten a tumor’s life. Cancer Res.74, 3199–3204 (2014). PubMedGoogle Scholar
- Dall’Olio, F. & Trinchera, M. Epigenetic bases of aberrant glycosylation in cancer. Int. J. Mol. Sci.18, 998 (2017). PubMed CentralGoogle Scholar
- Ashkani, J. & Naidoo, K. J. Glycosyltransferase gene expression profiles classify cancer types and propose prognostic subtypes. Sci. Rep.6, 26451 (2016). CASPubMedPubMed CentralGoogle Scholar
- Dusoswa, S. A. et al. Glioblastomas exploit truncated O-linked glycans for local and distant immune modulation via the macrophage galactose-type lectin. Proc. Natl Acad. Sci. USA117, 3693–3703 (2020). CASPubMedPubMed CentralGoogle Scholar
- ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-cancer analysis of whole genomes. Nature578, 82–93 (2020). Google Scholar
- Zeng, J. et al. Promoters of human Cosmc and T-synthase genes are similar in structure, yet different in epigenetic regulation. J. Biol. Chem.290, 19018–19033 (2015). CASPubMedPubMed CentralGoogle Scholar
- Agrawal, P. et al. Mapping posttranscriptional regulation of the human glycome uncovers microRNA defining the glycocode. Proc. Natl Acad. Sci. USA111, 4338–4343 (2014). CASPubMedPubMed CentralGoogle Scholar
- Kurcon, T. et al. miRNA proxy approach reveals hidden functions of glycosylation. Proc. Natl Acad. Sci. USA112, 7327–7332 (2015). CASPubMedPubMed CentralGoogle Scholar
- Bhattacharyya, R., Bhaumik, M., Raju, T. S. & Stanley, P. Truncated, inactive N-acetylglucosaminyltransferase III (GlcNAc-TIII) induces neurological and other traits absent in mice that lack GlcNAc-TIII. J. Biol. Chem.277, 26300–26309 (2002). CASPubMedGoogle Scholar
- Matsumoto, K. et al. N-Glycan fucosylation of epidermal growth factor receptor modulates receptor activity and sensitivity to epidermal growth factor receptor tyrosine kinase inhibitor. Cancer Sci.99, 1611–1617 (2008). CASPubMedGoogle Scholar
- Agrawal, P. et al. A systems biology approach identifies FUT8 as a driver of melanoma metastasis. Cancer Cell31, 804–819.e7 (2017). CASPubMedPubMed CentralGoogle Scholar
- Okada, M. et al. Blockage of core fucosylation reduces cell-surface expression of PD-1 and promotes anti-tumor immune responses of T cells. Cell Rep.20, 1017–1028 (2017). CASPubMedGoogle Scholar
- Marcos, N. T. et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated Sialyl-Tn antigen. Cancer Res.64, 7050–7057 (2004). CASPubMedGoogle Scholar
- Sewell, R. et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J. Biol. Chem.281, 3586–3594 (2006). CASPubMedGoogle Scholar
- Tarp, M. A. & Clausen, H. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim. Biophys. Acta1780, 546–563 (2008). CASPubMedGoogle Scholar
- Kudelka, M. R., Ju, T., Heimburg-Molinaro, J. & Cummings, R. D. Simple sugars to complex disease — mucin-type O-glycans in cancer. Adv. Cancer Res.126, 53–135 (2015). CASPubMedPubMed CentralGoogle Scholar
- Sutherlin, M. E. et al. Expression of three UDP-N-acetyl-α- d -galactosamine:polypeptide GalNAc N-acetylgalactosaminyltransferases in adenocarcinoma cell lines. Cancer Res.57, 4744–4748 (1997). CASPubMedGoogle Scholar
- Freire, T. et al. UDP-N-acetyl- d -galactosamine:polypeptide N-acetylgalactosaminyltransferase 6 (ppGalNAc-T6) mRNA as a potential new marker for detection of bone marrow-disseminated breast cancer cells. Int. J. Cancer119, 1383–1388 (2006). CASPubMedGoogle Scholar
- Song, K.-H. et al. GALNT14 promotes lung-specific breast cancer metastasis by modulating self-renewal and interaction with the lung microenvironment. Nat. Commun.7, 13796 (2016). CASPubMedPubMed CentralGoogle Scholar
- Kitada, S. et al. Polypeptide N-acetylgalactosaminyl transferase 3 independently predicts high-grade tumours and poor prognosis in patients with renal cell carcinomas. Br. J. Cancer109, 472–481 (2013). CASPubMedPubMed CentralGoogle Scholar
- Lavrsen, K. et al. De novo expression of human polypeptide N-acetylgalactosaminyltransferase 6 (GalNAc-T6) in colon adenocarcinoma inhibits the differentiation of colonic epithelium. J. Biol. Chem.293, 1298–1314 (2018). CASPubMedGoogle Scholar
- Dalziel, M. et al. The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J. Biol. Chem.276, 11007–11015 (2001). CASPubMedGoogle Scholar
- Swindall, A. F. & Bellis, S. L. Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J. Biol. Chem.286, 22982–22990 (2011). CASPubMedPubMed CentralGoogle Scholar
- Holdbrooks, A. T., Britain, C. M. & Bellis, S. L. ST6Gal-I sialyltransferase promotes tumor necrosis factor (TNF)-mediated cancer cell survival via sialylation of the TNF receptor 1 (TNFR1) death receptor. J. Biol. Chem.293, 1610–1622 (2018). CASPubMedGoogle Scholar
- Barthel, S. R. et al. α1,3 Fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc. Natl Acad. Sci. USA106, 19491–19496 (2009). CASPubMedPubMed CentralGoogle Scholar
- Esposito, M. et al. Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol.21, 627–639 (2019). CASPubMedPubMed CentralGoogle Scholar
- Engle, D. D. et al. The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice. Science364, 1156–1162 (2019). CASPubMedPubMed CentralGoogle Scholar
- Guo, H. et al. O-Linked N-acetylglucosamine (O-GlcNAc) expression levels epigenetically regulate colon cancer tumorigenesis by affecting the cancer stem cell compartment via modulating expression of transcriptional factor MYBL1. J. Biol. Chem.292, 4123–4137 (2017). CASPubMedPubMed CentralGoogle Scholar
- Hanover, J. A., Chen, W. & Bond, M. R. O-GlcNAc in cancer: an oncometabolism-fueled vicious cycle. J. Bioenerg. Biomembr.50, 155–173 (2018). CASPubMedGoogle Scholar
- Theocharis, A. D. & Karamanos, N. K. Proteoglycans remodeling in cancer: underlying molecular mechanisms. Matrix Biol.75–76, 220–259 (2019). PubMedGoogle Scholar
- Salanti, A. et al. Targeting human cancer by a glycosaminoglycan binding malaria protein. Cancer Cell28, 500–514 (2015). This paper identifies a broadly expressed cancer-associated 4-O-sulfated chondroitin sulfate epitope using the malarial receptor VAR2CSA. CASPubMedPubMed CentralGoogle Scholar
- Chen, Y.-H. et al. The GAGOme: a cell-based library of displayed glycosaminoglycans. Nat. Methods15, 881–888 (2018). This paper describes the development of a cell-based display of glycosaminoglycans. CASPubMedGoogle Scholar
- Petrosyan, A. Unlocking Golgi: why does morphology matter? Biochemistry84, 1490–1501 (2019). CASPubMedGoogle Scholar
- Kulkarni-Gosavi, P., Makhoul, C. & Gleeson, P. A. Form and function of the Golgi apparatus: scaffolds, cytoskeleton and signalling. FEBS Lett.593, 2289–2305 (2019). CASPubMedGoogle Scholar
- Petrosyan, A. Onco-Golgi: is fragmentation a gate to cancer progression? Biochem. Mol. Biol. J.https://doi.org/10.21767/2471-8084.100006 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Chia, J., Goh, G. & Bard, F. Short O-GalNAc glycans: regulation and role in tumor development and clinical perspectives. Biochim. Biophys. Acta1860, 1623–1639 (2016). CASPubMedGoogle Scholar
- Gill, D. J., Clausen, H. & Bard, F. Location, location, location: new insights into O-GalNAc protein glycosylation. Trends Cell Biol.21, 149–158 (2011). CASPubMedGoogle Scholar
- Axelsson, M. A. B. et al. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology11, 633–644 (2001). CASPubMedGoogle Scholar
- Chatterjee, S. et al. Protein paucimannosylation is an enriched N-glycosylation signature of human cancers. Proteomics19, e1900010 (2019). PubMedGoogle Scholar
- Chia, J., Tay, F. & Bard, F. The GalNAc-T Activation (GALA) pathway: drivers and markers. PLoS ONE14, e0214118 (2019). CASPubMedPubMed CentralGoogle Scholar
- Nguyen, A. T. et al. Organelle specific O-glycosylation drives MMP14 activation, tumor growth, and metastasis. Cancer Cell32, 639–653.e6 (2017). CASPubMedGoogle Scholar
- Yamaji, T. et al. A CRISPR screen using subtilase cytotoxin identifies SLC39A9 as a glycan-regulating factor. iScience15, 407–420 (2019). CASPubMedPubMed CentralGoogle Scholar
- Narimatsu, Y. et al. A validated gRNA library for CRISPR/Cas9 targeting of the human glycosyltransferase genome. Glycobiology28, 295–305 (2018). CASPubMedGoogle Scholar
- Narimatsu, Y. et al. An atlas of human glycosylation pathways enables display of the human glycome by gene engineered cells. Mol. Cell75, 394–407.e5 (2019). This paper describes the development and utility of a cell-based display of the human glycome. CASPubMedPubMed CentralGoogle Scholar
- Yang, Z. et al. Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat. Biotechnol.33, 842–844 (2015). CASPubMedGoogle Scholar
- Patnaik, S. K. & Stanley, P. Lectin-resistant CHO glycosylation mutants. Methods Enzymol.416, 159–182 (2006). CASPubMedGoogle Scholar
- Steentoft, C. et al. Precision genome editing: a small revolution for glycobiology. Glycobiology24, 663–680 (2014). CASPubMedGoogle Scholar
- Jae, L. T. et al. Deciphering the glycosylome of dystroglycanopathies using haploid screens for lassa virus entry. Science340, 479–483 (2013). CASPubMedPubMed CentralGoogle Scholar
- Han, J. et al. Genome-wide CRISPR/Cas9 screen identifies host factors essential for influenza virus replication. Cell Rep.23, 596–607 (2018). CASPubMedPubMed CentralGoogle Scholar
- Ye, L. et al. In vivo CRISPR screening in CD8 T cells with AAV–Sleeping Beauty hybrid vectors identifies membrane targets for improving immunotherapy for glioblastoma. Nat. Biotechnol.37, 1302–1313 (2019). CASPubMedPubMed CentralGoogle Scholar
- Miller, R. L. et al. Shotgun ion mobility mass spectrometry sequencing of heparan sulfate saccharides. Nat. Commun.11, 1481 (2020). CASPubMedPubMed CentralGoogle Scholar
- Stopschinski, B. E. et al. Specific glycosaminoglycan chain length and sulfation patterns are required for cell uptake of tau versus α-synuclein and β-amyloid aggregates. J. Biol. Chem.293, 10826–10840 (2018). CASPubMedPubMed CentralGoogle Scholar
- Tian, S. et al. Genome-wide CRISPR screens for Shiga toxins and ricin reveal Golgi proteins critical for glycosylation. PLoS Biol.16, e2006951 (2018). PubMedPubMed CentralGoogle Scholar
- Dabelsteen, S. et al. Essential functions of glycans in human epithelia dissected by a CRISPR–Cas9-engineered human organotypic skin model. Dev. Cell54, 669–684 (2020). PubMedPubMed CentralGoogle Scholar
- Büll, C., Joshi, H. J., Clausen, H. & Narimatsu, Y. Cell-based glycan arrays — a practical guide to dissect the human glycome. STAR Protoc.1, 100017 (2020). PubMedPubMed CentralGoogle Scholar
- Naegeli, A. & Aebi, M. Current approaches to engineering N-linked protein glycosylation in bacteria. Methods Mol. Biol.1321, 3–16 (2015). PubMedGoogle Scholar
- Hudak, J. E. & Bertozzi, C. R. Glycotherapy: new advances inspire a reemergence of glycans in medicine. Chem. Biol.21, 16–37 (2014). CASPubMedGoogle Scholar
- Van Landuyt, L., Lonigro, C., Meuris, L. & Callewaert, N. Customized protein glycosylation to improve biopharmaceutical function and targeting. Curr. Opin. Biotechnol.60, 17–28 (2019). PubMedGoogle Scholar
- Montero-Morales, L. & Steinkellner, H. Advanced plant-based glycan engineering. Front. Bioeng. Biotechnol.6, 81 (2018). PubMedPubMed CentralGoogle Scholar
- Walsh, G. Post-translational modifications of protein biopharmaceuticals. Drug Discov. Today15, 773–780 (2010). CASPubMedGoogle Scholar
- Čaval, T., Tian, W., Yang, Z., Clausen, H. & Heck, A. J. R. Direct quality control of glycoengineered erythropoietin variants. Nat. Commun.9, 3342 (2018). PubMedPubMed CentralGoogle Scholar
- Grabowski, G. A. et al. Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann. Intern. Med.122, 33–39 (1995). CASPubMedGoogle Scholar
- Umaña, P., Jean-Mairet, J., Moudry, R., Amstutz, H. & Bailey, J. E. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol.17, 176–180 (1999). PubMedGoogle Scholar
- Yamane-Ohnuki, N. et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol. Bioeng.87, 614–622 (2004). CASPubMedGoogle Scholar
- Meuris, L. et al. GlycoDelete engineering of mammalian cells simplifies N-glycosylation of recombinant proteins. Nat. Biotechnol.32, 485–489 (2014). CASPubMedPubMed CentralGoogle Scholar
- Chang, M. M. et al. Small-molecule control of antibody N-glycosylation in engineered mammalian cells. Nat. Chem. Biol.15, 730–736 (2019). CASPubMedGoogle Scholar
- Schulz, M. A. et al. Glycoengineering design options for IgG1 in CHO cells using precise gene editing. Glycobiology28, 542–549 (2018). CASPubMedGoogle Scholar
- Tian, W. et al. The glycosylation design space for recombinant lysosomal replacement enzymes produced in CHO cells. Nat. Commun.10, 1785 (2019). PubMedPubMed CentralGoogle Scholar
- RodrÍguez, E., Schetters, S. T. T. & van Kooyk, Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat. Rev. Immunol.18, 204–211 (2018). PubMedGoogle Scholar
- Mathiesen, C. B. K. et al. Genetically engineered cell factories produce glycoengineered vaccines that target antigen-presenting cells and reduce antigen-specific T-cell reactivity. J. Allergy Clin. Immunol.142, 1983–1987 (2018). CASPubMedGoogle Scholar
- Byrne, G. et al. CRISPR/Cas9 gene editing for the creation of an MGAT1-deficient CHO cell line to control HIV-1 vaccine glycosylation. PLoS Biol.16, e2005817 (2018). PubMedPubMed CentralGoogle Scholar
- Zhang, R. et al. Reducing immunoreactivity of porcine bioprosthetic heart valves by genetically-deleting three major glycan antigens, GGTA1/β4GalNT2/CMAH. Acta Biomater.72, 196–205 (2018). CASPubMedGoogle Scholar
- Liu, Q. P. et al. Identification of a GH110 subfamily of α1,3-galactosidases: novel enzymes for removal of the α3Gal xenotransplantation antigen. J. Biol. Chem.283, 8545–8554 (2008). CASPubMedPubMed CentralGoogle Scholar
- Ibrahim, A. M. S. et al. Acellular dermal matrices in breast surgery: a comprehensive review. Ann. Plast. Surg.70, 732–738 (2013). CASPubMedGoogle Scholar
- Neelamegham, S. et al. Updates to the Symbol Nomenclature for Glycans guidelines. Glycobiology29, 620–624 (2019). CASPubMedPubMed CentralGoogle Scholar
- Parker, J. L. & Newstead, S. Gateway to the Golgi: molecular mechanisms of nucleotide sugar transporters. Curr. Opin. Struct. Biol.57, 127–134 (2019). CASPubMedPubMed CentralGoogle Scholar
- Nabi, I. R., Shankar, J. & Dennis, J. W. The galectin lattice at a glance. J. Cell Sci.128, 2213–2219 (2015). CASPubMedGoogle Scholar
- Ilver, D. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science279, 373–377 (1998). CASPubMedGoogle Scholar
- Mahdavi, J. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science297, 573–578 (2002). CASPubMedPubMed CentralGoogle Scholar
- Rossez, Y. et al. The LacdiNAc-specific adhesin LabA mediates adhesion of Helicobacter pylori to human gastric mucosa. J. Infect. Dis.210, 1286–1295 (2014). CASPubMedGoogle Scholar
- Nagae, M. et al. Structure and mechanism of cancer-associated N-acetylglucosaminyltransferase-V. Nat. Commun.9, 3380 (2018). PubMedPubMed CentralGoogle Scholar
- Pinho, S. S. & Reis, C. A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer15, 540–555 (2015). CASPubMedGoogle Scholar
- Läubli, H. & Varki, A. Sialic acid-binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses. Cell. Mol. Life Sci.77, 593–605 (2020). PubMedGoogle Scholar
- Maxson, J. E. et al. Ligand-independence of the colony stimulating factor 3 receptor (CSF3R) T618I mutation results from loss of O-linked glycosylation and increased receptor dimerization. J. Biol. Chem.289, 5820–5827.(2014). CASPubMedPubMed CentralGoogle Scholar
- Jiang, Y. et al. O-glycans on death receptors in cells modulate their sensitivity to TRAIL-induced apoptosis through affecting on their stability and oligomerization. FASEB J.34, 11786–11801 (2020). CASPubMedGoogle Scholar
- Rillahan, C. D. & Paulson, J. C. Glycan microarrays for decoding the glycome. Annu. Rev. Biochem.80, 797–823 (2011). CASPubMedPubMed CentralGoogle Scholar
- Blixt, O. et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl Acad. Sci. USA101, 17033–17038 (2004). CASPubMedPubMed CentralGoogle Scholar
Acknowledgements
The authors are grateful to R. Schnaar and B. Henrissat for discussions and critical comments on the manuscript. They thank H. Wandall, A. Halim, C. Büll, Y. Zhang and L. Hansen for help with the manuscript, and all members of the Copenhagen Center for Glycomics for discussions. Supported by the Lundbeck Foundation, the Novo Nordisk Foundation and the Danish National Research Foundation (DNRF107).









